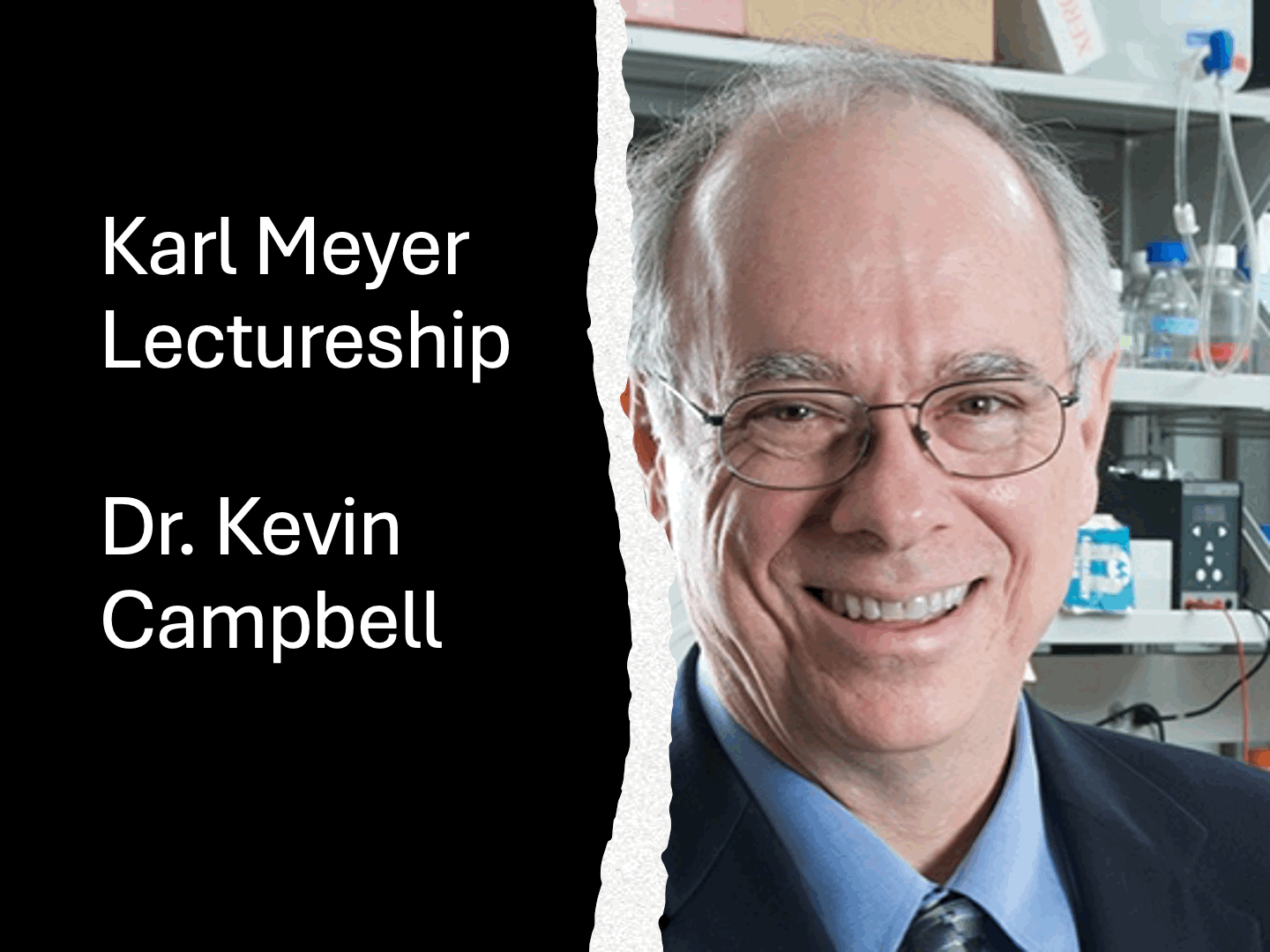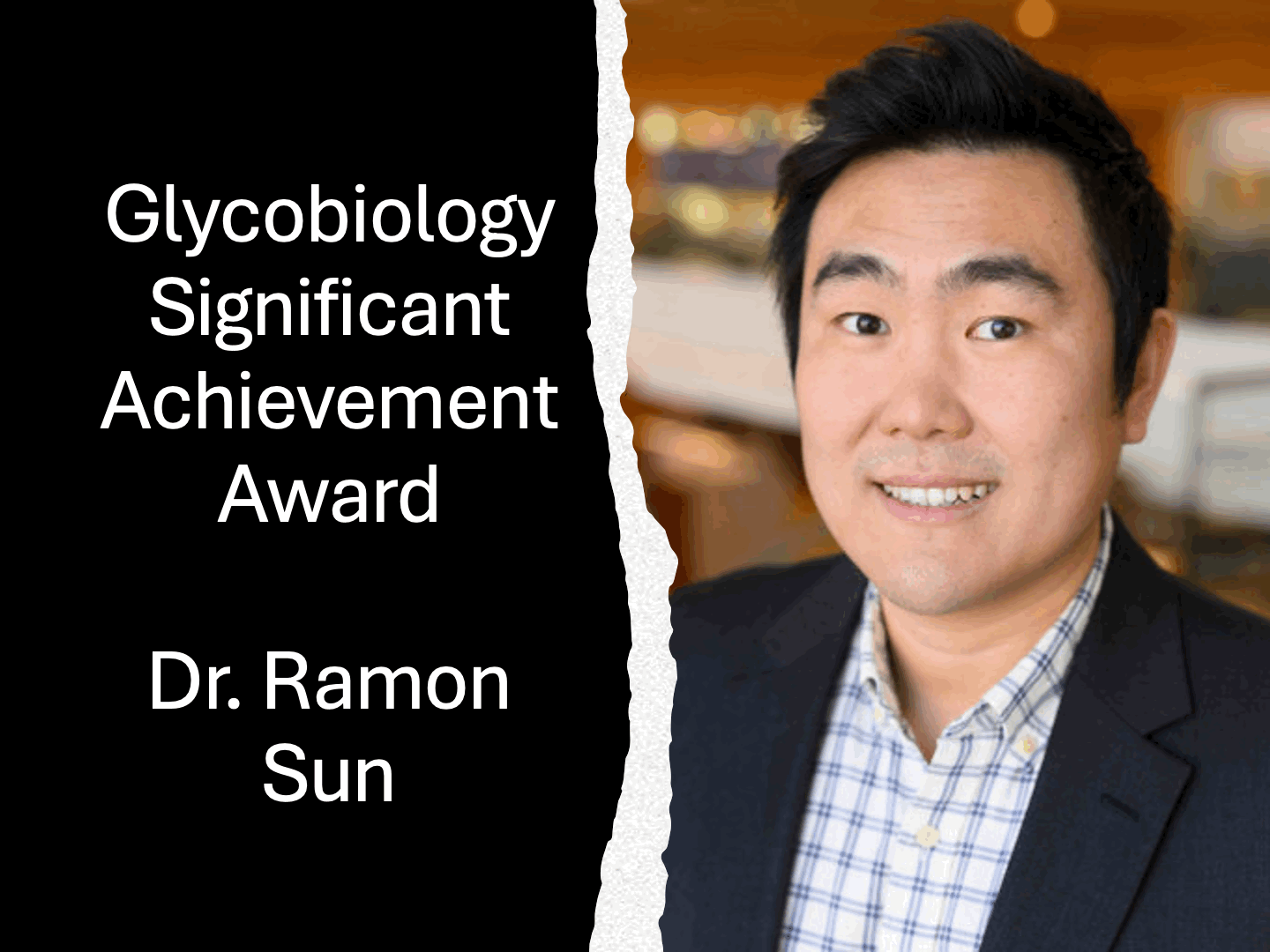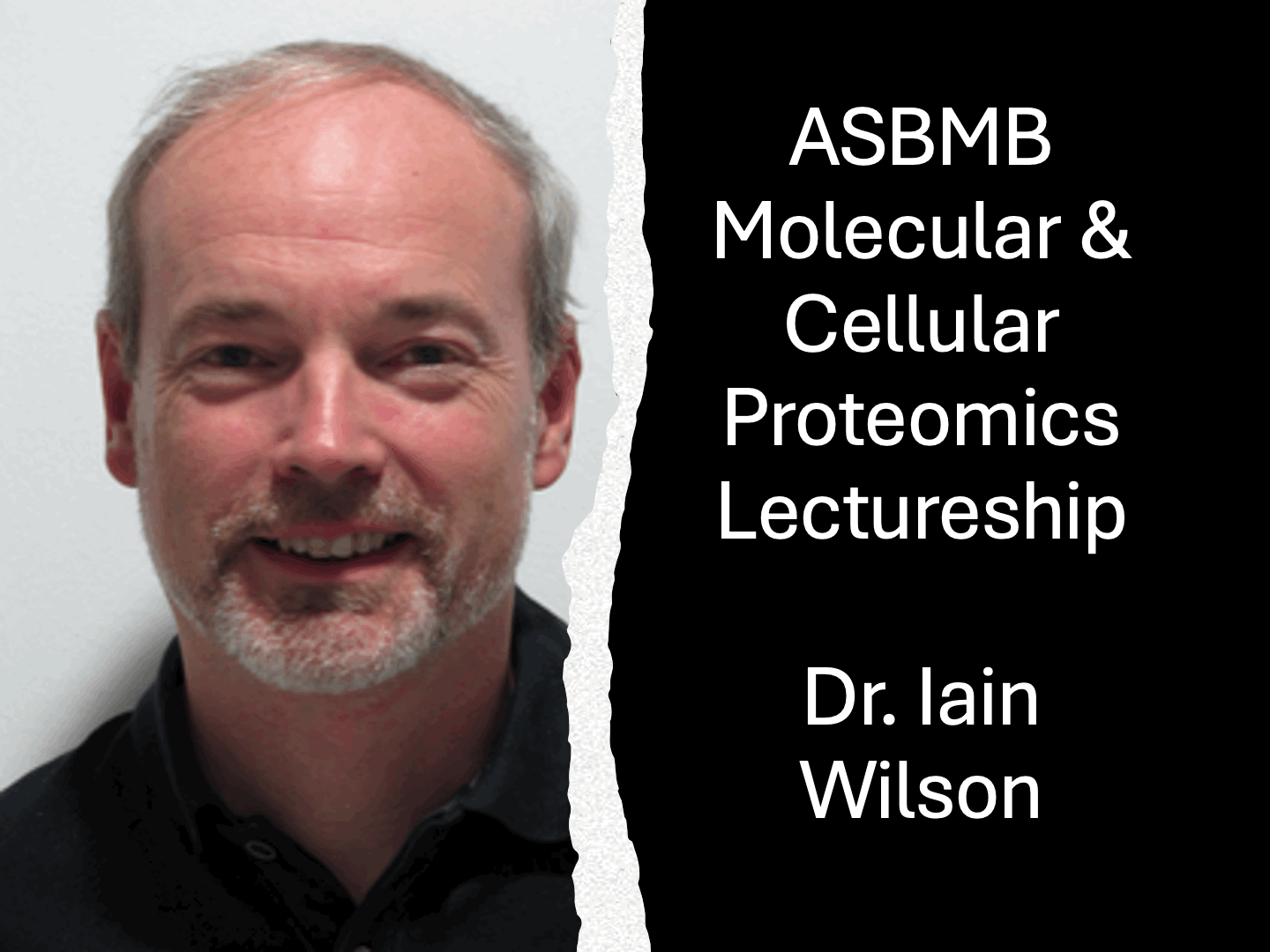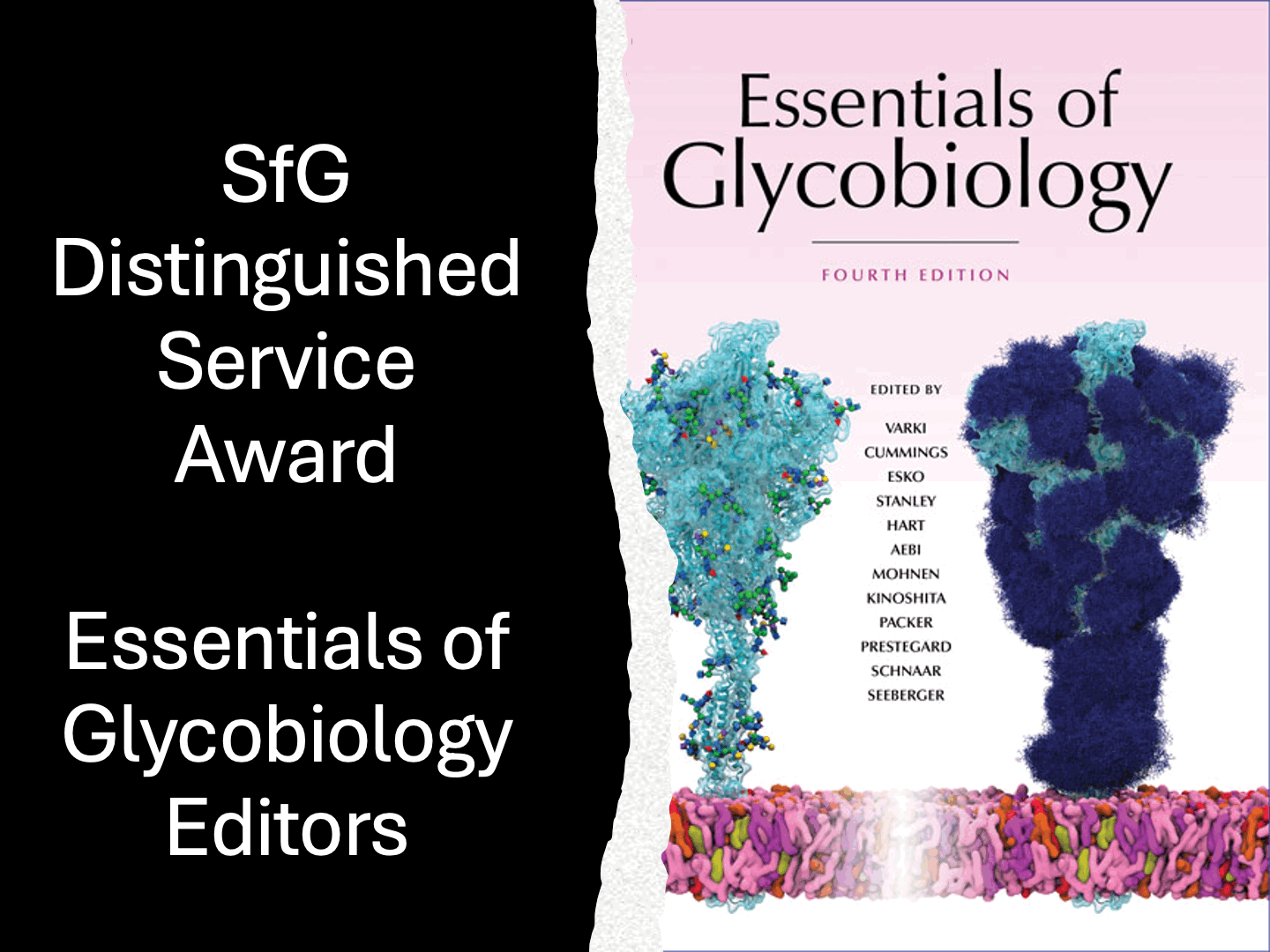Dr. Ten Feizi wins 2014 Rosalind Kornfeld AwardThe Society for Glycobiology is pleased to announce that the recipient of the 2014 Rosalind Kornfeld Award is awarded to Dr Ten Feizi. The Rosalind Kornfeld Award for Lifetime Achievement in Glycobiology was established in 2008 to honor the distinguished scientific career and service to the Society by Dr. Rosalind Kornfeld. The award is given by the Society to scientists who have, over their professional lifetimes, made significant contributions with important impact on the field.
Professor Feizi received her medical training and began her research career at the Royal Free Hospital in London. Following fellowships with Elvin Kabat at the College of Physicians and Surgeons of Columbia University and Henry and Kunkel at the Rockefeller University, she returned to the United Kingdom in 1973. She developed her research group at the Medical Research Council Clinical Research Centre, which has evolved over the years into what is now the Glycosciences Laboratory of Imperial College London. Study of glycans and proteins that recognize them have been intimately intertwined with each other throughout Professor Feizi's career. Her initial studies of cold agglutinins, in particular monoclonal autoantibodies (anti-I) that increase following Mycoplasma pneumoniae infection, led to analysis of the I blood group antigen which she showed to be expressed on carbohydrate backbones of the major blood group antigens. Collaboration with Sen-itiroh Hakomori, a previous recipient of this award, resulted in elucidation of the structures of the Ii blood group antigens as branched and linear poly-N-acetyllactosamine chains, in parallel with analysis of the different specificities of the antibodies that react preferentially with one structure or the other. She went on to demonstrate the utility of the anti-Ii blood group antibodies and other anti-carbohydrate antibodies as tools for following changes in glycosylation during normal cell differentiation and in the transformation of normal cells into tumor cells. Embracing the evolving technology of monoclonal antibodies led to the demonstration that antibodies to developmental and tumor antigens were often specific for carbohydrate structures. A key feature of Professor Feizi's approach to the field has been that she is not only interested in the structure but also the function of glycans. Thus, as the evidence for changes in glycosylation was mounting, she began speculating on the role of these changes during development and tumorigenesis. Speculation led to experiments on the ability of the newly emerging class of endogenous animal lectins to bind to cell surface oligosaccharides, initially focusing on the galectins. She also returned to the relationship between the I blood group antigen and mycoplasma, demonstrating that a sialylated form of the poly-N-acetyllactosamine chains is the target receptor for the mycoplasma. These examples provide fundamental paradigms in glycobiology, in which mammalian cell surface glycans serve as ligands for endogenous glycan-binding receptors as well as targets for pathogen binding. Because of her interest both in the structures of glycans and in their recognition, Professor Feizi saw the need for novel approaches to screening for target glycans for antibodies and the newly emerging family of animal lectins. Combining her expertise in isolation and analysis of sugars with her understanding of sugar-binding proteins, she developed the neoglycolipid approach to displaying the whole spectrum of glycans, of specific cells and glycoproteins and interrogating them with antibodies and receptors. The advantages of such panels displaying what we would now call the glycome, compared to testing one ligand at a time, were quickly demonstrated by a series of studies from Professor Feizi's group. Complementing natural libraries of this type with panels of chemically defined oligosaccharides provided the foundation for the glycan array approach. This key breakthrough, which has been developed and refined in her laboratory over the past 30 years, is one of the key technologies underlying the current field of glycobiology. The approach has been instrumental both to defining the specificities of receptors and antibodies for specific sugar structures and for identifying which sugars attached to glycoprotein counter-receptors are the functional targets of the glycan-binding proteins. As the power of the neoglycolipid array approach has become apparent, a queue of illustrious investigators have beaten a path to Professor Feizi's door, leading to wide-ranging collaborations on the specificity of practically every class of glycan-binding receptor. For her contributions both to transformational methodology and to the conceptual framework of the field of glycobiology, Professor Ten Feizi is an outstanding recipient of the 2014 Rosalind Kornfeld Award. |

 Dr. Ten Feizi (Glycosciences Laboratory, Imperial College London) is the recipient of the 2014 Rosalind Kornfeld Award for Lifetime Achievement in Glycobiology, given by the Society of Glycobiology in recognition of her many achievements in the fields of structure analysis, immunology and function of glycans over nearly 50 years.
Dr. Ten Feizi (Glycosciences Laboratory, Imperial College London) is the recipient of the 2014 Rosalind Kornfeld Award for Lifetime Achievement in Glycobiology, given by the Society of Glycobiology in recognition of her many achievements in the fields of structure analysis, immunology and function of glycans over nearly 50 years.