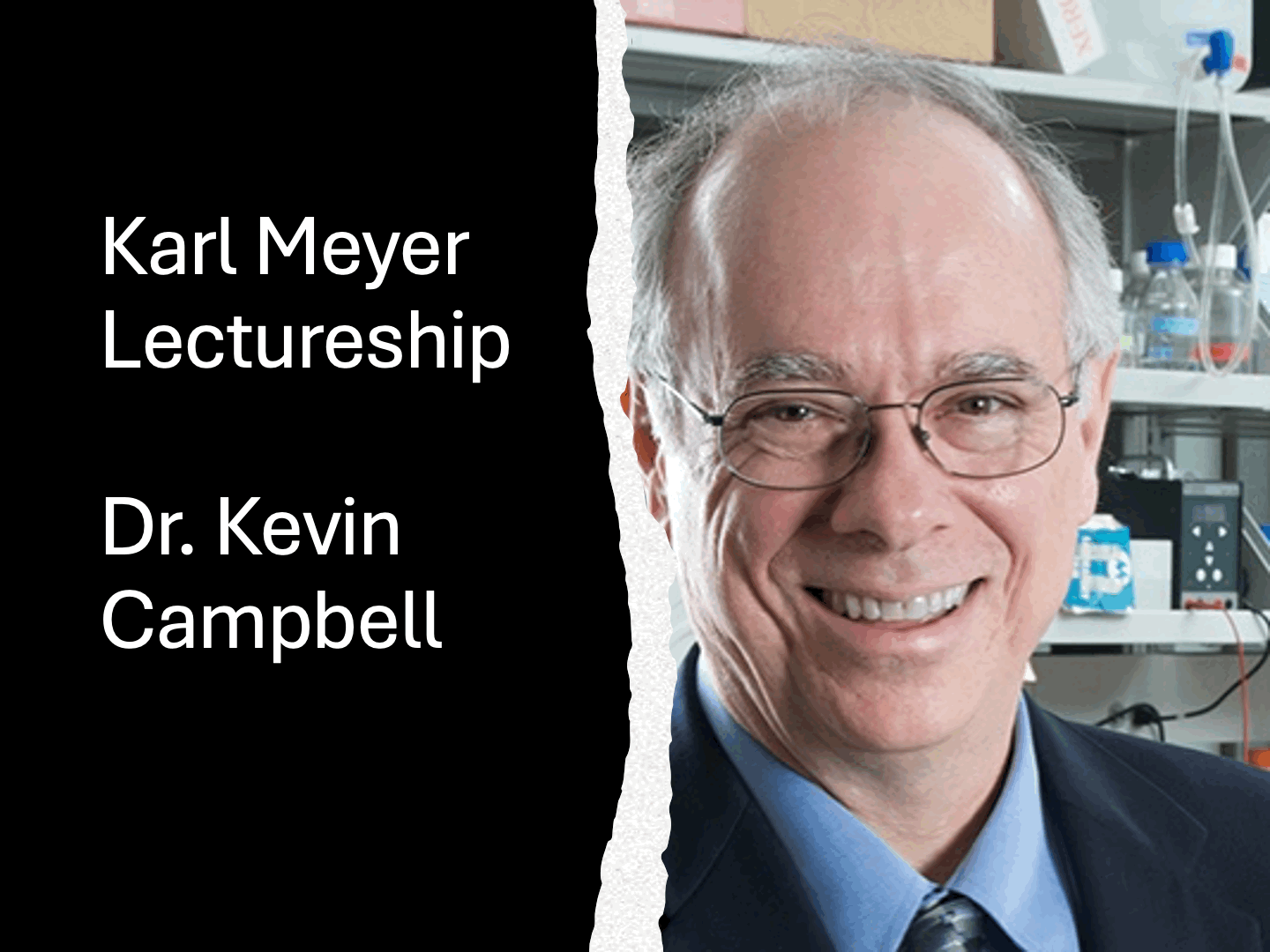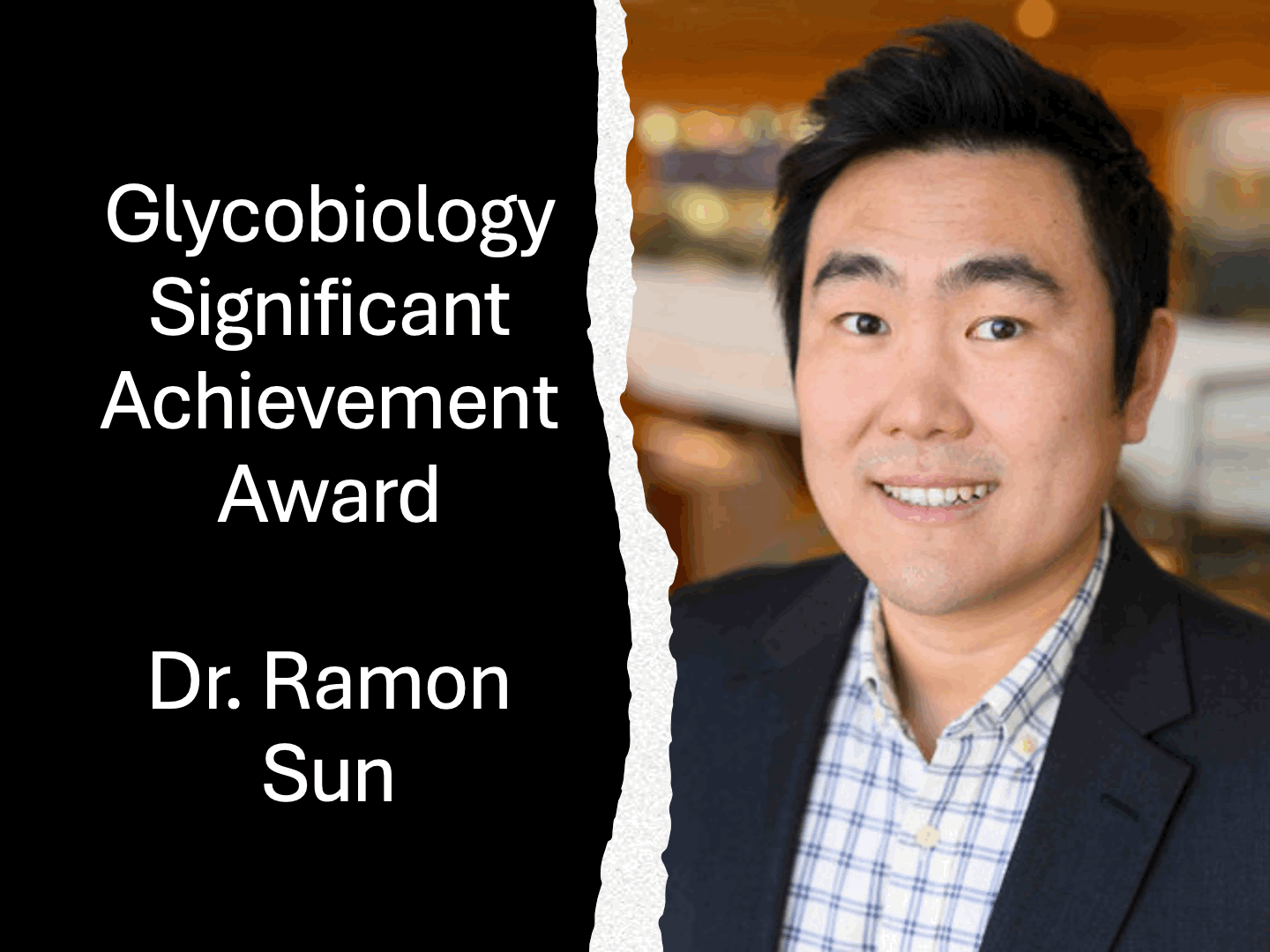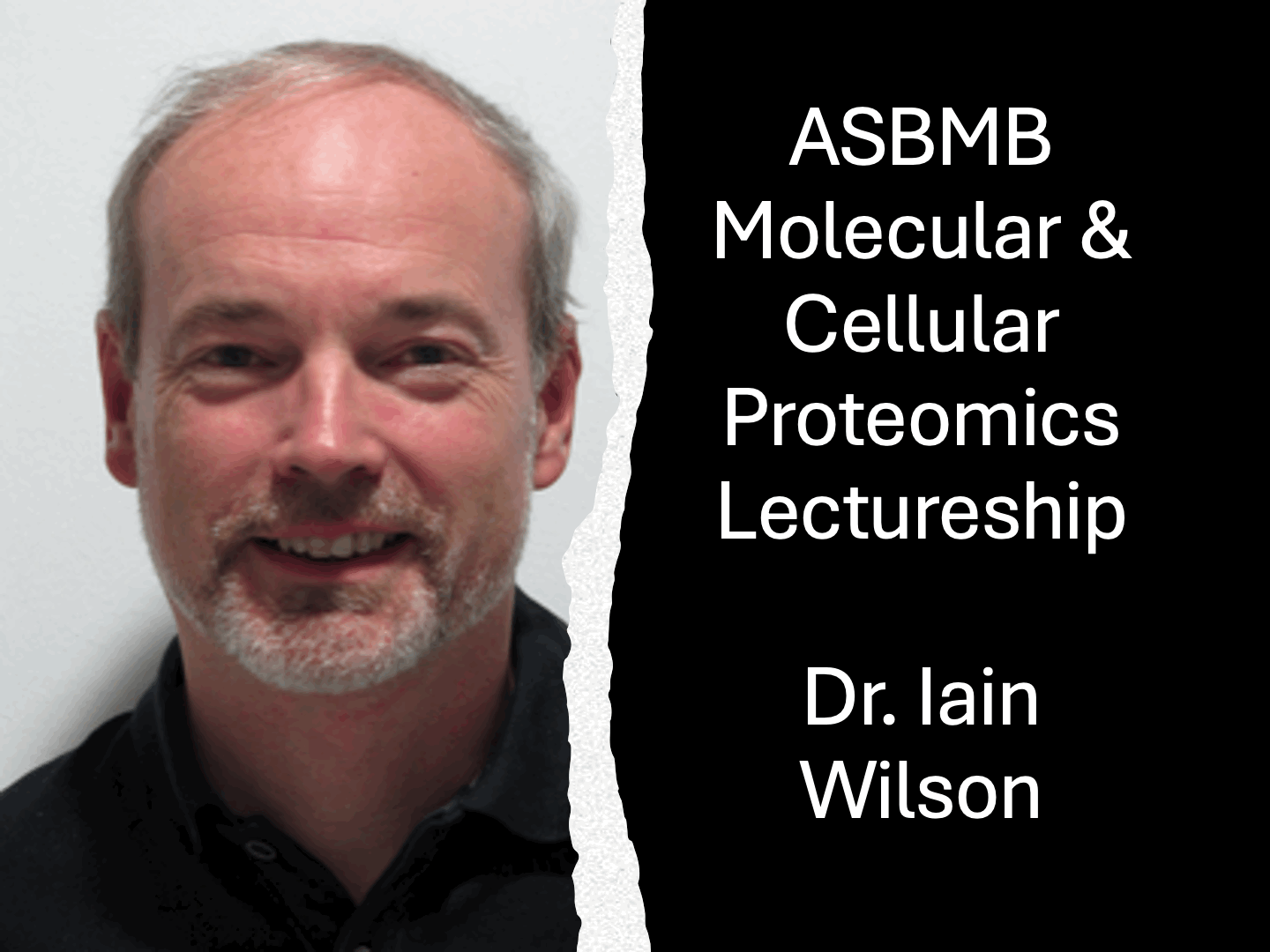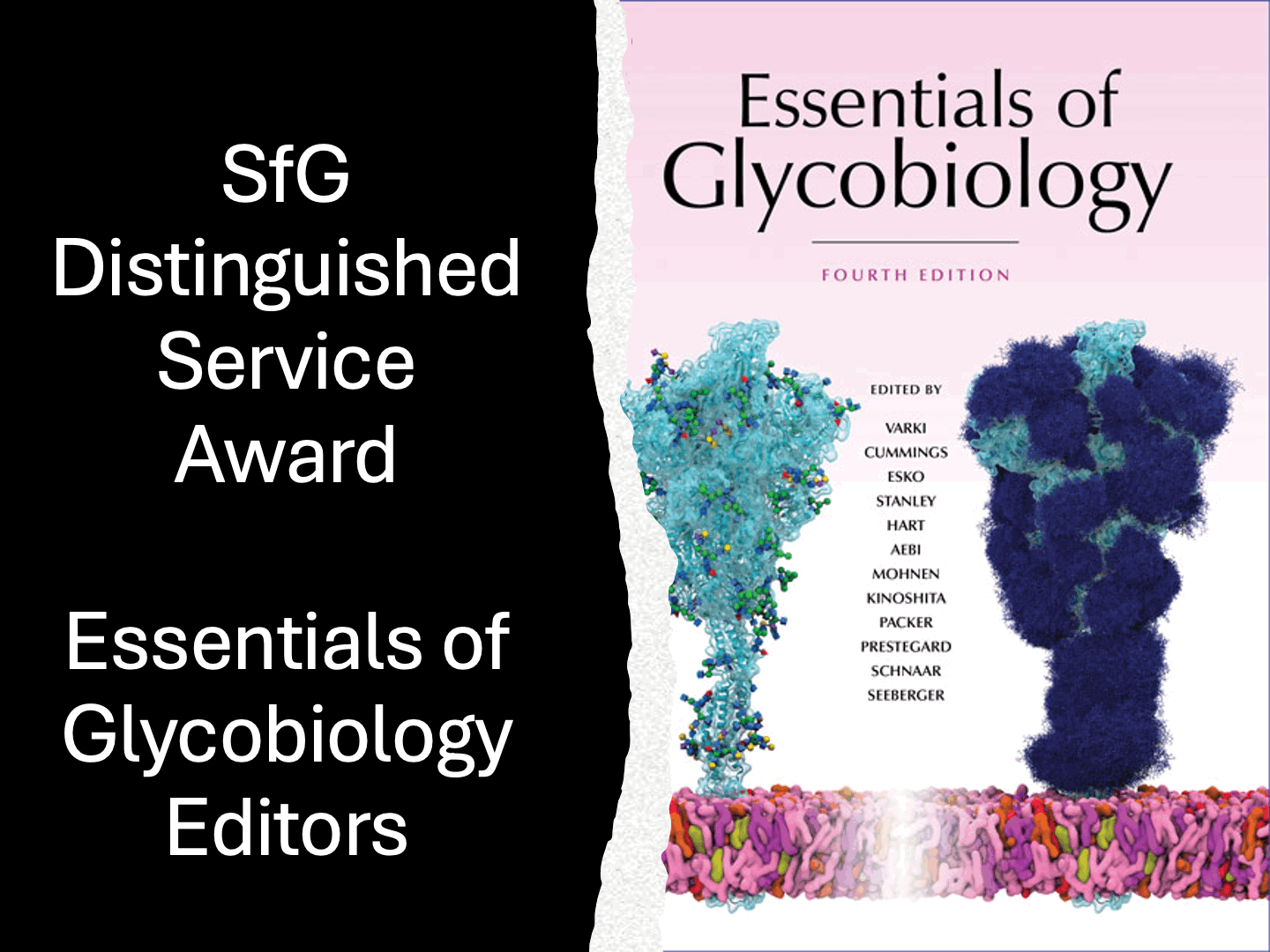Karl Meyer Lectureship Award
In 1990, the Society established the Karl Meyer Lectureship Award to honor the distinguished career of Karl Meyer and his outstanding contributions to the field of Glycobiology. This international award is presented at the Annual Meeting of the Society to a well-established scientist with a currently active research program who has made widely recognized major contributions to the field of Glycobiology. 2024 Awardee - Dr. Kevin Campbell
The 2024 Karl Meyer Award will be presented to Dr. Kevin Campbell, Investigator, Howard Hughes Medical Institute, Director, Wellstone Muscular Dystrophy Specialized Research Center, Professor and Chair, Department of Molecular Physiology and Biophysics, University of Iowa. Dr. Campbell discovered dystroglycan, a novel high affinity extracellular matrix receptor required for skeletal muscle function in the 1990’s. His laboratory cloned dystroglycan and elucidated its function as an extracellular matrix receptor in skeletal muscle. He demonstrated that disrupted dystroglycan expression is a key feature in the pathogenesis of Duchenne muscular dystrophy. Subsequently, his biochemical and physiological studies showed that α-dystroglycan binds with high-affinity to laminin, a component of the extracellular matrix that surrounds muscle cells; that the C-terminus of β-dystroglycan anchors dystrophin to the membrane; and that dystrophin binds actin filaments. His group also showed binding of laminin to α-dystroglycan enables the extracellular matrix to strengthen the muscle cell membrane, thus maintaining the integrity of the skeletal muscle sarcolemma. Collectively, this work illustrated the mechanism by which dystroglycan links the subsarcolemmal actin cytoskeleton to the extracellular matrix that surrounds skeletal muscle and prevents muscular dystrophy. His laboratory went on to discover defects in dystroglycan glycosylation disrupting the functional link between the cytoskeleton and extracellular matrix cause Walker-Warburg syndrome, muscle-eye-brain disease, and Fukuyama congenital muscular dystrophy, all congenital muscular dystrophies with associated defects in brain development. Specifically, his laboratory showed O-mannosylation of dystroglycan is abnormal in each of these three distinct congenital diseases and abnormal O-glycosylation of dystroglycan disrupts its normal binding to each of its major extracellular matrix ligands in muscle and brain: laminin, neurexin, and agrin. He also deduced from mouse studies that the functional disruption of dystroglycan underlies the pathogenesis of developmental brain abnormalities associated with congenital muscular dystrophies. Dr. Campbell’s efforts to understand the structural basis underlying laminin binding to dystroglycan led him to identify like-acetylglucosaminyltransferase (LARGE1), a novel bifunctional enzyme with both xylosyltransferase and glucuronyltransferase activities. He showed LARGE1 synthesizes matriglycan, a polysaccharide comprised of alternating glucuronic acid (GlcA) and xylose (Xyl) residues. Thus, his work revealed a novel repeating disaccharide in mammals, which Dr. Meyer surely would have appreciated. His laboratory also made major strides in defining the M3 O-mannose underlying matriglycan. Importantly, this included identifying a glycosylation-specific O-mannose kinase, an SGK196 “pseudokinase” now termed POMK, which is required for synthesis of functional α-dystroglycan. His laboratory continues to utilize mouse models, structural biology, and classical biochemistry to further define the role of matriglycan, O-mannosylation, and α-dystroglycan as scaffolds for the ECM that ensure proper skeletal muscle function and prevent muscular dystrophy. For his pioneering scientific contributions, Kevin has received numerous awards and honors, including being an investigator of the Howard Hughes Medical Institute since 1989, receiving both the Amgen (1994) and Herbert Tabor Research (2020) Awards from the American Society for Biochemistry and Molecular Biology, being elected as a member of the National Academy of Medicine (1999) and National Academy of Sciences, and most recently (2024) being named a Fellow of the American Association for the Advancement of Science. Kevin also received the Tamio Yamakawa Award from the Japan Consortium for Glycobiology and Glycotechnology in 2020 and the SFG President’s Innovator Award in 2017. In summary, Kevin is a true trailblazer and role model in the field of glycobiology, and the 2024 Karl Meyer Lectureship Award recognizes his seminal contributions not only to our basic understanding of functional glycosylation of α-dystroglycan, but also to the appreciation of how defects in the O-mannose pathway translate into congenital muscular dystrophies. |

 The Karl Meyer Lectureship Award was established in 1990 to honor the distinguished career of Karl Meyer and his outstanding contributions to the field of Glycobiology. This international award is given to well-established scientists with currently active research programs who have made widely recognized major contributions to the field of Glycobiology.
The Karl Meyer Lectureship Award was established in 1990 to honor the distinguished career of Karl Meyer and his outstanding contributions to the field of Glycobiology. This international award is given to well-established scientists with currently active research programs who have made widely recognized major contributions to the field of Glycobiology.